What
What is lithium?
Astronomer Carl Sagan has said that “We are made of star stuff”.
In that great nuclear furnace that forged us there were three key elements, the first to be born after the Big Bang: traces of a heavier version of hydrogen called deuterium, a lighter type of helium, and a small amount of lithium.
Today, according to scientific discoveries, it abounds on the surface of young stars and meteorites in the Solar System. On Earth, 85% of lithium reserves are concentrated in Chilean, Bolivian and Argentine territory.
It is a highly reactive metallic, sodium-like, alkaline chemical element that is not found in a free state in nature. In order for it to be used in the pharmaceutical industry or in electromobility, it must undergo a complex process, which requires state-of-the-art technology, and then be treated. This is what SQM does in the Salar de Atacama, in Chile.
Although very light, lithium -with the symbol Li and atomic number 3- is an excellent conductor of heat and electricity. It is found mainly in natural brines, pegmatites, oil wells, geothermal fields, and seawater.
What
Relevance of lithium
If you wear glasses, walk on ceramic tiles, or turn on a computer, you are interacting with products that use lithium to make them.
Being one of the metals with the highest energy density, it is a material that is widely used in the technology area.
Its electrochemical potential makes it ideal for the anode – positive pole – of electric batteries; it is also very important for pharmaceutical companies and for industrial uses.
Reserves
52% of the world’s lithium deposits are found in Chile, specifically in the Salar de Atacama. SQM is one of the leading companies in its sustainable extraction and commercialization.
By 2025, around 800 thousand tons of Lithium Carbonate Equivalent (LCE) will be needed worldwide, which means US $ 6 billion in sales.
LCE is the salt already subjected to the purification processes and represents the most used unit to measure supply and demand. Chile alone has calculated reserves of 7.5 million tons of lithium, 9.3 times the estimated world demand.

Present in our lives
In 1817, Swedish chemist Johann A. Arfvedson discovered the existence of lithium. In subsequent years, it was used to treat everything from diseases such as gout to psychiatric disorders. Today, it is still essential in the treatment of bipolar disorder, but it is a key element in many other things.
Lithium batteries power our phones, tablets, cars, among many other everyday devices. It is applied in the production of heat resistant glass, lubricating greases, adhesives, and telescopes. When you walk on concrete streets, on that surface you step on, there is lithium.
It is also present in functions that are essential for life, not only on the earth’s surface: to extract carbon dioxide from the air, lithium hydroxide is used in submarines and spacecraft.
Its uses are so versatile that in 2019, NASA Expedition 59 flight engineers Nick Hague and Anne McClain made a six-hour and 39-minute spacewalk on the International Space Station (ISS) to replace nickel-hydrogen batteries with newer and more powerful lithium-ion batteries in one of the channels that supplies the energy derived from the ISS solar panels.
What
Electric vehicles
Currently, lithium ion is the main technology used in electric car batteries internationally and it is projected that the demand for this material will continue to grow.
This element offers a number of advantages: it does not cause energy pollution as does fossil-fuel combustion, nor does it produce noise pollution, since the noise of the electric motor is much lower than that of traditional vehicles.
Lithium thus contributes to sustainable development.
What
Fresh water and brine: why they are different
In the Salar de Atacama, brines naturally formed which, for the extraction of lithium, are naturally favored by the high geothermal gradient of the sector. They are pools that are saturated with sodium chloride and high concentrations of potassium, magnesium, boron, sulfates and lithium. Although water is also found in these basins, it is not suitable for human or animal consumption.
Why? Imagine a glass of fresh water, one of sea water and one of brine. The former has a maximum of 1,500 mg/l of total dissolved solids (TDS) and is, therefore, drinkable. If you have up to 5,000 mg/l TDS, it cannot be drunk, but it is suitable for irrigation. Seawater has 35,000 mg/l TDS. It could, after several treatments, be desalinated and transformed into a form suitable for human consumption.
Brine, on the other hand, is much more complex: it has a TDS concentration of more than 300,000 mg/l, 200 times more than drinking water and 70 times more than the worst-quality water for irrigation, in addition to other minerals. That is why it can be said that the extraction of elements from the brines of the Salar de Atacama does not directly affect the freshwater resources of the area.
What
Water care
SQM uses a very marginal percentage of fresh water compared to traditional mining operation: 180 l/s of fresh water for the operations in the Salar de Atacama, specifically to help the processes of pumping brine and potassium production.
These 180 l/s represent less than 5% of the groundwater rights granted by the authority to the company and less than 2.5% of the total rights granted by the authority in the basin to the company, if we also consider the concession of surface water. They are used for the production in total of more than two million tons of potassium salts (MOP, sylvinites and carnallites), 270 thousand tons of concentrated solution of lithium chloride and magnesium salts (bischofite).
The water channeling is carried out from wells located to the east of the Atacama Salt Flat, as well as the brine extractions. Both are governed by an Environmental Impact Study (EIS) and are a priority in the company’s monitoring and early warning plans.
The greatest water consumption in the area comes from the copper mining companies, which have rights for more than 2,800 l/s, almost double the amount of brine extracted by SQM.
SQM’s lithium hydroxide production consumes ~24 l/kg of water directly in its production processes in Salar de Atacama (Pöyry 2020, Life Cycle Assessment (LCA) for Lithium Products), therefore, 2/3 of the water is used for the production of potassium and its salts. In comparison, cotton consumes ~400X and beef ~571X more water than SQM’s lithium hydroxide production process in the Salar de Atacama.
What
MAIN LITHIUM COMPOUNDS
The most relevant are
- Lithium carbonate, from which organic and inorganic compounds such as lithium bromide, lithium fluoride and lithium nitrate are derived.
- Lithium hydroxide, from which HP lithium carbonate and lithium peroxide are derived.
- Lithium chloride.
- Metallic lithium.
MAIN LITHIUM COMPOUNDS
Lithium carbonate
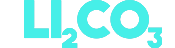
This is a salt that is obtained after purification of lithium. It is currently the most widely used solution and it is projected to represent around 42% of the lithium market by 2027.
It is used in the pharmaceutical industry for psychiatric treatments; in the ceramic and glass manufacturing processes, and as a cathode material in lithium ion batteries.
SQM is therefore present when you turn on a laptop, talk on a mobile phone or use a digital camera.
MAIN LITHIUM COMPOUNDS
Lithium hydroxide

The spacecraft from NASA’s Mercury, Gemini and Apollo projects used lithium hydroxide as an absorbent, as it readily captures carbon dioxide from water vapor. The possibilities offered by this compound, which is mainly used in the production of lubricating greases capable of operating in extreme conditions of temperature and load, are as broad and fascinating as can be.
Approximately 70% of the lubricating greases produced in the world contain lithium. It is also used in batteries and dyes.
LITHIUM HYDROXIDE
Long-life batteries
Lithium hydroxide possesses chemical and physical properties that are key to achieving electrochemical performance and longevity in high nickel content (60%) batteries for electric cars.
These types of cathodes have the advantage of a longer service life and allow longer distances on a single charge.
LITHIUM HYDROXIDE
Lubricating greases
This is the most used thickening agent in multipurpose greases for automotive and industrial lubrication.
It is characterized by its high anti-corrosion power, long life and resistance to wear. In addition, it has a strong adhesion to any surface and high resistance in conditions of extreme heat or cold, -20ºC to + 120ºC, and humidity.
For these reasons, it leads the world’s grease production with 70%, but that’s not all: lithium is also an important ingredient in making agricultural applications that help protect the food we grow and eat.
LITHIUM HYDROXIDE
Automotive market and electromobility
The electric vehicle market has been gaining more and more relevance in the world, displacing internal combustion engines that operate using fossil fuels.
Really impressive growth is expected in the coming years: 60 million electric cars are projected to be sold by 2040, mainly in Europe and Asia. This is in line with the recommendations of the International Energy Agency which estimates that, to meet the climate objectives of the Paris Accords, at least 40% of vehicle sales must be electric models.
The adoption of this new technology is being driven by governments through state subsidies and incentives. Consumers, for their part, are motivated to participate in reducing global greenhouse gas emissions.
Another relevant factor is the new generation of advanced batteries, which will allow greater autonomy. In fact, most major original equipment manufacturers have announced the development of self-driving fleets.
MAIN LITHIUM COMPOUNDS
LITHIUM CHLORIDE

It is used in automobile solder, as a desiccant for drying air currents. In more specialized applications, it is applied to organic synthesis, for example, as an additive in the Stille reaction. In biochemistry, it can be used to precipitate RNA from cell extracts.
Also used as a flame colorant to produce dark red flames.
MAIN LITHIUM COMPOUNDS
METALLIC LITHIUM

This is used in the aerospace industry.

